- Home Page
- Company Profile
-
Our Products
- Castor Oil Derivatives
- Cetyl Ricinoleate Supplier
- Ethyl Undecanoate
- Heptanoic Acid
- Methyl Undecanoate - Exporter
- Zinc Ricinoleate Exporter
- Glyceryl Monoricinoleate Exporter
- Glyceryl Mono Undecylenate - Exporter
- Heptyl Undecylenate Supplier
- Methyl Ricinoleate - Manufacturer
- Polyricinoleic Acid - Manufacturer
- Pentaerythritol Mono Ricinoleate- Supplier
- Ricinoleic Acid - Manufacturer
- Undecanoic Acid 98% Min. By GC
- Alcohol C-11 Undecylenic
- Methyl Undecylenate 98% Min. Purity By GC
- Undecylenic Acid
- Zinc Undecylenate as per BP/EP/USP
- Butyl Ricinoleate - Manufacturer
- Calcium Ricinoleate
- Calcium Undecylenate as per USP
- Heptaldehyde 97% Min. By GC
- Heptyl Alcohol 98%Min.By GC
- Methyl Acetyl Ricinoleate - Supplier
- Aldehyde C11 Undecylenic
- Butyl Undecylenate
- Castor Oil
- Condensed Ricinoleic Acid
- Ethyl Undecylenate
- Glyceryl Mono Undecenoate - Castor Oil Derivative
- Sodium Undecylenate
- Chromatographic Adsorbents
- Fatty Acid Esters & Salts
- Zinc Ricinoleate Supplier
- Methyl Oleate - Supplier
- Cetyl Ricinoleate Manufacturer
- Glyceryl Monoricinoleate Supplier
- Glyceryl Mono Undecylenate Manufacturer
- Heptyl Undecylenate Manufacturer
- Methyl Ricinoleate
- Pentaerythritol Mono Ricinoleate
- Butyl Oleate 99% Min. By GC
- Methyl Undecylenate - Supplier
- Butyl Ricinoleate
- Methyl Acetyl Ricinoleate - Manufacturer
- Butyl Oleate Commercial
- Methyl Oleate Commercial- Supplier
- Behenic Acid Methyl Ester
- Caprylic Acid
- Erucic Acid
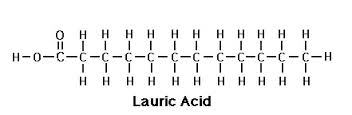
- Lauric Acid
- Methyl Heptanoate
- Oleic Acid Ethyl Ester
- Palmitic Acid
- Potassium Oleate
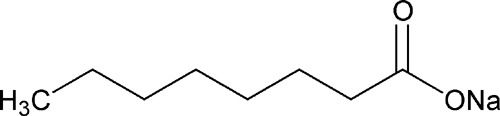
- Sodium Caprylate - Supplier
- Soya Oil Methyl Ester
- Stearic Acid
- Arachidic Acid Ethyl Ester - Supplier
- Elaidic Acid Methyl Ester
- Glyceryl Mono Undecenoate - Supplier
- Linoleic Acid Methyl Ester 99% Min. By GC
- Methyl Undecanoate
- Palmitic Acid Methyl Ester
- Sodium Oleate
- Potassium Caprylate
- Stearic Acid Methyl Ester
- Behenic Acid Ethyl Ester
- Linoleic Acid Ethyl Ester
- Fatty Alcohols
- Lipid Chemicals
- Arachidic Acid 99% Min By GC
- Elaidic Acid 99% Min. By GC
- Linoleic Acid 99% Min. By GC
- Methyl Oleate 99% Min. By GC
- Oleic Acid 99% Min.By GC
- Methyl Oleate Commercial
- Arachidic Acid Methyl Ester
- Arachidic Acid Ethyl Ester
- Elaidyl Alcohol - Manufacturer
- Linoleyl Alcohol - Manufacturer
- Linoleic Acid Ethyl Ester - Manufacturer
- Odor Absorbers
- Ricinoleates
- Zinc Ricinoleate Manufacturer
- Cetyl Ricinoleate Exporter
- Glyceryl Monoricinoleate Manufacturer
- Methyl Ricinoleate - Supplier
- Pentaerythritol Mono Ricinoleate- Manufacturer
- Polyricinoleic Acid
- Ricinoleic Acid
- Butyl Ricinoleate - Supplier
- Calcium Ricinoleate - Manufacturer
- Methyl Acetyl Ricinoleate - Exporter
- Cosmetic Ingredients
- Undecylenic Acid - Cosmetics
- Zinc Ricinoleate
- Potassium Caprylate - Cosmetics
- Cetyl Ricinoleate
- Glyceryl Monoricinoleate
- Heptyl Undecylenate
- Undecanoic Acid - Cosmetics
- Undecylenic Monoethanolamide - Cosmetics
- Zinc Undecylenate - Cosmetics
- Methyl Acetyl Ricinoleate
- Aldehyde C11 Undecylenic - Cosmetics
- Arachidyl Alcohol - Cosmetics
- Behenic Acid Methyl Ester - Cosmetics
- Caprylic Acid - Cosmetics
- Erucic Acid - Cosmetics
- Lauric Acid - Cosmetics
- Methyl Heptanoate - Cosmetics
- Oleic Acid Ethyl Ester - Cosmetics
- Oleyl Alcohol - Cosmetics
- Palmitic Acid - Cosmetics
- Potassium Oleate - Cosmetics
- Sodium Caprylate - Cosmetics
- Soya Oil Methyl Ester - Cosmetics
- Stearic Acid - Cosmetics
- Butyl Undecylenate - Cosmetics
- Castor Oil - Cosmetics
- Glyceryl Mono Undecenoate - Cosmetics
- Heptanoic Acid - Cosmetics
- Linoleic Acid Methyl Ester - Cosmetics
- Palmitic Acid Methyl Ester - Cosmetics
- Sodium Oleate - Cosmetics
- Sodium Undecylenate - Cosmetics
- Stearic Acid Methyl Ester - Cosmetics
- Linoleic Acid Ethyl Ester - Cosmetics
- Emollient
- Cetyl Ricinoleate - Cosmetics
- Glyceryl Mono Undecylenate-Cosmetics
- Glyceryl Monoricinoleate - Cosmetics
- Heptyl Undecylenate - Cosmetics
- Methyl Acetyl Ricinoleate - Cosmetics
- Erucic Acid - Emollient
- Oleic Acid Ethyl Ester - Manufacturer
- Palmitic Acid - Exporter
- Ethyl Undecylenate - Manufacturer
- Glyceryl Mono Undecenoate - Manufacturer
- Palmitic Acid Methyl Ester - Supplier
- Stearic Acid Methyl Ester - Exporter
- Personal Care Ingredients
- Cetyl Ricinoleate - Personal Care Product
- Glyceryl Monoricinoleate- Personal Care Product
- Heptyl Undecylenate - Personal Care Product
- Methyl Acetyl Ricinoleate - Personal Care Product
- Aldehyde C11 Undecylenic - Exporter
- Arachidyl Alcohol - Manufacturer
- Behenic Acid Methyl Ester - Manufacture
- Caprylic Acid - Manufacturer
- Lauric Acid - Personal Care Product
- Oleic Acid Ethyl Ester - Personal Care Product
- Oleyl Alcohol - Manufacturer
- Palmitic Acid - Manufacturer
- Potassium Oleate - Manufacturer
- Soya Oil Methyl Ester - Manufacturer
- Stearic Acid - Manufacturer
- Palmitic Acid Methyl Ester - Manufacturer
- Sodium Oleate - Supplier
- Sodium Undecylenate - Supplier
- Natural Preservatives
- Undecylenates
- Glyceryl Mono Undecylenate-Supplier
- Heptyl Undecylenate Exporter
- Undecanoic Acid 98% Min. By GC - Manufacturer
- Sodium Undecylenate - Manufacturer
- Methyl Undecylenate 98% Min. By GC - Manufacturer
- Undecylenic Acid - Manufacturer
- Zinc Undecylenate - Manufacturer
- Calcium Undecylenate - Manufacturer
- Butyl Undecylenate - Supplier
- Castor Oil - Manufacturer
- Ethyl Undecylenate - Supplier
- Glyceryl Mono Undecenoate - Exporter
- Skin Care Products
- Glyceryl Mono Undecylenate- Skin Care Product
- Zinc Ricinoleate- Skin Care Product
- Glyceryl Monoricinoleate- Skin Care Product
- Heptyl Undecylenate - Skin Care Product
- Calcium Ricinoleate - Supplier
- Methyl Acetyl Ricinoleate - Skin Care Product
- Cetyl Ricinoleate - Skin Care Product
- Aldehyde C11 Undecylenic - Supplier
- Arachidyl Alcohol - Supplier
- Behenic Acid Methyl Ester - Supplier
- Caprylic Acid - Supplier
- Oleic Acid Ethyl Ester - Skin Care Product
- Oleyl Alcohol - Exporter
- Palmitic Acid - Supplier
- Potassium Oleate - Supplier
- Soya Oil Methyl Ester - Supplier
- Stearic Acid - Supplier
- Castor Oil - Supplier
- Glyceryl Mono Undecenoate - Skin Care Product
- Palmitic Acid Methyl Ester - Exporter
- Stearic Acid Methyl Ester - Manufacturer
- Emulsifier
- Glyceryl Monoricinoleate - Emulsifier
- Glyceryl Mono Undecylenate - Emulsifier
- Zinc Ricinoleate - Emulsifier
- Ricinoleic Acid - Emulsifier
- Undecanoic Acid - Emulsifier
- Linoleic Acid - Emulsifier
- Methyl Oleate - Emulsifier
- Oleic Acid - Emulsifier
- Undecylenic Acid - Emulsifier
- Heptaldehyde - Emulsifier
- Silica Gel for Column Chromatography - Emulsifier
- Arachidic Acid - Emulsifier
- Methyl Oleate Commercial- Emulsifier
- Caprylic Acid - Emulsifier
- Lauric Acid - Emulsifier
- Methyl Heptanoate - Emulsifier
- Oleyl Alcohol - Emulsifier
- Palmitic Acid - Emulsifier
- Potassium Oleate - Emulsifier
- Sodium Caprylate - Emulsifier
- Stearic Acid - Emulsifier
- Castor Oil - Emulsifier
- Glyceryl Mono Undecenoate - Emulsifier
- Linoleic Acid Methyl Ester - Emulsifier
- Palmitic Acid Methyl Ester - Emulsifier
- Sodium Oleate - Emulsifier
- Potassium Caprylate - Emulsifier
- Sodium Undecylenate - Emulsifier
- Stearic Acid Methyl Ester - Emulsifier
- Hair Care Products
- Heptyl Undecylenate- Hair Care Product
- Zinc Ricinoleate- Hair Care Product
- Butyl Oleate - Hair Care Product
- Linoleic Acid - Hair Care Product
- Butyl Oleate Commercial -Supplier
- Oleic Acid - Supplier
- Undecylenic Acid - Supplier
- Arachidic Acid - Hair Care Product
- Lauric Acid - Exporter
- Oleic Acid Ethyl Ester - Supplier
- Oleyl Alcohol - Supplier
- Stearic Acid - Exporter
- Sodium Undecylenate - Exporter
- Stearic Acid Methyl Ester - Supplier
- Lubricants
- Glyceryl Monoricinoleate - Lubricant
- Heptyl Undecylenate Lubricant
- Methyl Ricinoleate - Lubricant
- Polyricinoleic Acid - Lubricant
- Pentaerythritol Mono Ricinoleate - Lubricant
- Ricinoleic Acid - Lubricant
- Butyl Oleate - Lubricant
- Butyl Oleate Commercial- Lubricant
- Methyl Undecylenate - Lubricant
- Butyl Ricinoleate - Lubricant
- Methyl Acetyl Ricinoleate - Lubricant
- Behenic Acid Methyl Ester - Lubricant
- Caprylic Acid - Lubricant
- Erucic Acid - Lubricant
- Lauric Acid - Lubricant
- Oleyl Alcohol - Lubricant
- Potassium Oleate - Lubricant
- Soya Oil Methyl Ester - Lubricant
- Stearic Acid - Lubricant
- Castor Oil - Lubricant
- Heptanoic Acid - Lubricant
- Linoleic Acid Methyl Ester - Lubricant
- Palmitic Acid Methyl Ester - Lubricant
- Sodium Oleate - Lubricant
- Stearic Acid Methyl Ester - Lubricant
- Textile Chemicals
- Leather Chemicals
- Pharma Ingredients
- Zinc Undecylenate - Pharma Ingredient
- Undecylenic Acid - Pharma Ingredient
- Caprylic Acid - Pharma Ingredient
- Undecylenic Monoethanolamide
- Calcium Undecylenate - Pharma Ingredient
- Lauric Acid - Pharma Ingredient
- Sodium Caprylate - Manufacturer
- Castor Oil - Pharma Ingredient
- Heptanoic Acid - Supplier
- Sodium Oleate - Manufacturer
- Anti Fungals
- Undecylenic Acid - Antifungal
- Zinc Undecylenate - Anti Fungal
- Undecanoic Acid - Anti Fungal
- Undecylenic Monoethanolamide - Anti Fungal
- Calcium Ricinoleate - Anti Fungal
- Calcium Undecylenate - Anti Fungal
- Caprylic Acid - Anti Fungal
- Lauric Acid - Anti Fungal
- Sodium Caprylate - Anti Fungal
- Castor Oil - Anti Fungal
- Nail Care Product
- Separation Aids
- Perfumery Chemicals
- Alcohol C-11 Undecylenic - Supplier
- Methyl Undecylenate - Exporter
- Heptaldehyde - Manufacturer
- Heptyl Alcohol - Supplier
- Ethyl Undecylenate - Exporter
- Aldehyde C11 Undecylenic - Manufacturer
- Caprylic Acid - Exporter
- Methyl Heptanoate - Supplier
- Oleic Acid Ethyl Ester - Exporter
- Butyl Undecylenate - Manufacturer
- Castor Oil - Perfumery Chemical
- Ethyl Undecanoate - Manufacturer
- Linoleic Acid Methyl Ester - Exporter
- Heptanoic Acid - Manufacturer
- Methyl Undecanoate - Manufacturer
- Linoleic Acid Ethyl Ester - Exporter
- Fragrance Chemicals
- Alcohol C-11 Undecylenic - Exporter
- Heptyl Alcohol - Exporter
- Butyl Oleate - Fragrance Chemical
- Butyl Oleate Commercial - Manufacturer
- Methyl Undecylenate - Perfume & Fragrance Chemical
- Heptaldehyde - Supplier
- Aldehyde C11 Undecylenic - Perfumes & Fragrances
- Caprylic Acid - Perfumes & Fragrances
- Methyl Heptanoate - Perfumes & Fragrances
- Oleic Acid Ethyl Ester - Perfumes & Fragrances
- Castor Oil - Fragrance Chemical
- Ethyl Undecanoate - Supplier
- Ethyl Undecylenate - Perfumes & Fragrances
- Heptanoic Acid - Exporter
- Linoleic Acid Methyl Ester - Supplier
- Methyl Undecanoate - Supplier
- Linoleic Acid Ethyl Ester - Perfumes & Fragrances
- Nutritional Supplements
- Arachidic Acid - Pharmaceutical Chemical
- Elaidic Acid - Pharmaceutical Chemical
- Linoleic Acid - Pharmaceutical Chemical
- Methyl Oleate - Pharmaceutical Chemical
- Oleic Acid - Pharmaceutical Chemical
- Arachidic Acid Methyl Ester - Supplier
- Elaidyl Alcohol - Supplier
- Lauric Acid - Supplier
- Linoleyl Alcohol - Exporter
- Methyl Heptanoate - Manufacturer
- Oleic Acid Ethyl Ester - Pharmaceutical Chemical
- Oleyl Alcohol - Pharmaceutical Chemical
- Sodium Caprylate
- Linoleic Acid Methyl Ester - Manufacturer
- Specialty Fatty Acid
- Arachidic Acid - Manufacturer
- Elaidic Acid - Manufacturer
- Linoleic Acid - Manufacturer
- Methyl Oleate - Manufacturer
- Oleic Acid - Manufacturer
- Methyl Oleate Commercial - Manufacturer
- Arachidic Acid Methyl Ester - Manufacturer
- Arachidic Acid Ethyl Ester - Manufacturer
- Elaidyl Alcohol - Exporter
- Lauric Acid - Manufacturer
- Linoleyl Alcohol - Supplier
- Linoleic Acid Ethyl Ester - Supplier
- Aldehydes
- Silica Gel for Column Chromatography
- Silica Gel for Thin Layer Chroamtography
- Castor Oil Derivatives
- Contact Us
Undecylenic Acid - Pharma Ingredient
Product Details:
- Loss on Drying 0.5%
- Heavy Metal (%) 0.001%
- Ph Level 4.0-5.5 (10% solution in ethanol)
- Smell Characteristic fatty odor
- Taste Fatty, slightly bitter
- Molecular Formula C11H20O2
- Melting Point -24C
- Click to view more
X
Undecylenic Acid - Pharma Ingredient Product Specifications
- Not applicable (liquid form)
- 98% Min
- CH2=CH(CH2)8COOH
- Active Pharmaceutical Ingredient (API)
- Undecylenic Acid
- Insoluble in water; soluble in alcohol, ether, chloroform, fixed oils
- Clear to pale yellow liquid
- Colorless to pale yellow
- Store in a cool, dry, well-ventilated place, away from sources of ignition
- 112-38-9
- Pharma Grade
- 29161990
- 184.28 g/mol
- 203-964-4
- Non-poisonous at recommended pharmaceutical concentrations
- 268C
- Used as an antifungal agent, particularly in topical formulations
- -24C
- 24 months in unopened original packaging
- C11H20O2
- Fatty, slightly bitter
- Characteristic fatty odor
- 10-Undecenoic Acid
- 4.0-5.5 (10% solution in ethanol)
- 0.001%
- 0.5%
- Liquid
Product Description
|
Undecylenic Acid as per BP/EP/USP |
|
|
PARAMETERS |
SPECIFICATIONS |
|
Synonyms |
10-Undecenoic Acid, Hendecenoic acid |
|
CAS No. |
112-38-9 |
|
Formula |
C11H20O2. CH2= CH (CH2)8COOH |
|
Mol. Wt. |
184.28 |
|
Purity |
99% min by GLC. |
|
Appearance & Color |
Colorless to pale yellow liquid or leafy crystals |
|
Odor |
Variable from oily fruity to sour fatty |
|
B.P |
275-279 (decomposes). |
|
Occurence |
Not found in Nature. Synthetic Product. |
|
Acid Value |
296-305 |
|
Sp.Gr 25/25 |
0.91 average (liquid). |
|
Iodine Value |
135-140 |
|
Saponification Value |
296-305 |
|
Congealing Point |
23oC to 24oC |
|
Stability |
Stable when stored properly |
Acme Synthetic Chemicals is the Manufacturer, Supplier & also the Exporter of Undecylenic Acid as per BP/EP/USP
Undecylenic Acid - Pharma Ingredient is also known as 10- Undecenoic Acid , Hendecenoic Acid which is classified under CAS No. 112-38-9
Undecylenic Acid - Pharma Ingredient,10-Undecenoic acid, Hendecenoic acid ( CAS No.112-38-9) is produced by cracking of castor oil under pressure.
Undecylenic Acid - Pharma Ingredient ( CAS No.112-38-9) is a very versatile raw-material.
Undecylenic Acid - Pharma Igredient (CAS No.112-38-9) is used in the manufacture of synthetic nylons,"Nylon-11" which is an important engineering polymer.
Several perfumery bases can be prepared from Undecylenic Acid - Pharma Ingredient (CAS No.112-38-9) & its derivatives.
The following perfumery chemicals can be prepared from Undecylenic Acid - Pharma Ingredient (CAS No.112-38-9) & its derivatives.(Methyl undecylenate,Ethyl undecylenate,Allyl undecylenate,Undecylenic aldehyde,Undecylenic alcohol,Undecalactone (Aldehyde C-14).)
Undecylenic Acid - Pharma Ingredient (CAS No.112-38-9) is the starting material for other important aroma chemicals such as pelorgonic acid and esters, several Macrocylic Musks such as Ethylene Brassylate (Musk T) exaltone, exaltolide, Musk RI, Cevolide etc.
A variety of compounds useful as bactericides, fungicides & insect sprays are produced from Undecylenic Acid - Pharma Ingredient (CAS No.112-38-9).
Many pharmaceutical formulations use mixtures of Undecylenic Acid & its Zinc/ Calcium Salts as active ingredients for their anti-fungal properties in remedies for fungal skin infections such as atheletes foot, ringworm etc.
Ethoxylated Undecylenic Acid shows promise as an emulsifier that can also replace some or biocides in cosmetics. Several companies have incorporated this in their formulations.
Undecylenic Acid - Pharma Ingredient (CAS No.112-38-9) is also used in the synthesis of insect pheromones. Undecylenic Acid - Pharma Ingredient (CAS No.112-38-9) and its zinc soaps are used as fungicides in medicine and agriculture.
Undecylenic Acid - Pharma Ingredient ( CAS No.112-38-9) is used in the manufacture of pharmaceuticals, cosmetics and perfumery, including anti dandruff shampoos, antimicrobial powders and as a musk in perfumes and aromas.
Acmes current capacity for Undecylenic Acid - Pharma Ingredient (CAS No.112-38-9) is 1200 tons per annum.
Acme can offer Undecylenic Acid - Pharma Ingredient(CAS No.112-38-9) in drums / FCL Lots and ISO Tanks.
Undecylenic Acid - Pharma Ingredient (CAS No. 112-38-9) is packed in 210 kg HMHDPE ( plastic ) drums.
Each FCL can take 80 drums of Undecylenic Acid - Pharma Ingredient (CAS No.112-38-9).
An ISO tank can take between 18 to 20 tons of Undecylenic Acid - Pharma Ingredient (CAS No.112-38-9).
Applications & Usage
Undecylenic Acid is mainly used as an antifungal pharmaceutical ingredient, especially in skin creams, lotions, and ointments for topical applications. Its strong antifungal properties make it suitable for treating various fungal infections, such as athletes foot and ringworm. Its compatibility with a range of solvents and excipients also makes it a versatile choice in pharmaceutical formulations.
Quality, Safety & Benefits
This API is manufactured under strict quality control to ensure high purity (minimum 98%) and low impurity levels (1.0%). It is non-poisonous at recommended concentrations and carries a low peroxide and heavy metal content. Being compliant with international guidelines, it is safe for pharmaceutical use and offers a long shelf life. Its antifungal efficacy, stability, and compliance make it a reliable option for pharmaceutical manufacturers.
FAQs of Undecylenic Acid - Pharma Ingredient:
Q: How do I properly store Undecylenic Acid to maintain its quality?
A: Undecylenic Acid should be stored in a cool, dry, well-ventilated place away from sources of ignition. Keeping the product in its unopened original HDPE packaging helps preserve its quality and extends its shelf life up to 24 months.Q: What are the main pharmaceutical uses of Undecylenic Acid?
A: Undecylenic Acid is primarily used as an active pharmaceutical ingredient in topical antifungal treatments. It is effective against various fungal infections, including athletes foot and ringworm, due to its robust antifungal properties.Q: When should Undecylenic Acid be handled with extra caution?
A: Care should be taken during handling to avoid exposure to open flames or sources of ignition because of its flash point (126C, closed cup). Additionally, ensure proper ventilation and follow standard laboratory protective measures during use.Q: Where is Undecylenic Acid typically used in the pharmaceutical industry?
A: This ingredient is widely incorporated in the formulation of creams, ointments, powders, and sprays for external (topical) human use. It is especially favored for products targeting skin and nail fungal infections.Q: What benefits does Undecylenic Acid offer in pharmaceutical formulations?
A: It offers excellent antifungal efficacy, chemical stability, and is non-poisonous at therapeutic concentrations. Its compliance with strict quality standards and long shelf life make it highly beneficial for pharmaceutical manufacturers and end-users alike.Q: How is Undecylenic Acid supplied for commercial use?
A: The product is packaged in HDPE drums or as specified by the customer to ensure safe transportation and storage. This helps maintain product integrity and compliance with pharmaceutical regulations.Tell us about your requirement

Price:
Quantity
Select Unit
- 50
- 100
- 200
- 250
- 500
- 1000+
Additional detail
Mobile number
Email




 Call Me Free
Call Me Free
