- Home Page
- Company Profile
-
Our Products
- Castor Oil Derivatives
- Cetyl Ricinoleate Supplier
- Ethyl Undecanoate
- Heptanoic Acid
- Methyl Undecanoate - Exporter
- Zinc Ricinoleate Exporter
- Glyceryl Monoricinoleate Exporter
- Glyceryl Mono Undecylenate - Exporter
- Heptyl Undecylenate Supplier
- Methyl Ricinoleate - Manufacturer
- Polyricinoleic Acid - Manufacturer
- Pentaerythritol Mono Ricinoleate- Supplier
- Ricinoleic Acid - Manufacturer
- Undecanoic Acid 98% Min. By GC
- Alcohol C-11 Undecylenic
- Methyl Undecylenate 98% Min. Purity By GC
- Undecylenic Acid
- Zinc Undecylenate as per BP/EP/USP
- Butyl Ricinoleate - Manufacturer
- Calcium Ricinoleate
- Calcium Undecylenate as per USP
- Heptaldehyde 97% Min. By GC
- Heptyl Alcohol 98%Min.By GC
- Methyl Acetyl Ricinoleate - Supplier
- Aldehyde C11 Undecylenic
- Butyl Undecylenate
- Castor Oil
- Condensed Ricinoleic Acid
- Ethyl Undecylenate
- Glyceryl Mono Undecenoate - Castor Oil Derivative
- Sodium Undecylenate
- Chromatographic Adsorbents
- Fatty Acid Esters & Salts
- Zinc Ricinoleate Supplier
- Methyl Oleate - Supplier
- Cetyl Ricinoleate Manufacturer
- Glyceryl Monoricinoleate Supplier
- Glyceryl Mono Undecylenate Manufacturer
- Heptyl Undecylenate Manufacturer
- Methyl Ricinoleate
- Pentaerythritol Mono Ricinoleate
- Butyl Oleate 99% Min. By GC
- Methyl Undecylenate - Supplier
- Butyl Ricinoleate
- Methyl Acetyl Ricinoleate - Manufacturer
- Butyl Oleate Commercial
- Methyl Oleate Commercial- Supplier
- Behenic Acid Methyl Ester
- Caprylic Acid
- Erucic Acid
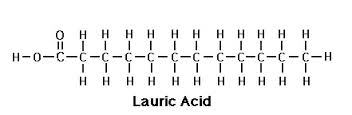
- Lauric Acid
- Methyl Heptanoate
- Oleic Acid Ethyl Ester
- Palmitic Acid
- Potassium Oleate
- Sodium Caprylate - Supplier
- Soya Oil Methyl Ester
- Stearic Acid
- Arachidic Acid Ethyl Ester - Supplier
- Elaidic Acid Methyl Ester
- Glyceryl Mono Undecenoate - Supplier
- Linoleic Acid Methyl Ester 99% Min. By GC
- Methyl Undecanoate
- Palmitic Acid Methyl Ester
- Sodium Oleate
- Potassium Caprylate
- Stearic Acid Methyl Ester
- Behenic Acid Ethyl Ester
- Linoleic Acid Ethyl Ester
- Fatty Alcohols
- Lipid Chemicals
- Arachidic Acid 99% Min By GC
- Elaidic Acid 99% Min. By GC
- Linoleic Acid 99% Min. By GC
- Methyl Oleate 99% Min. By GC
- Oleic Acid 99% Min.By GC
- Methyl Oleate Commercial
- Arachidic Acid Methyl Ester
- Arachidic Acid Ethyl Ester
- Elaidyl Alcohol - Manufacturer
- Linoleyl Alcohol - Manufacturer
- Linoleic Acid Ethyl Ester - Manufacturer
- Odor Absorbers
- Ricinoleates
- Zinc Ricinoleate Manufacturer
- Cetyl Ricinoleate Exporter
- Glyceryl Monoricinoleate Manufacturer
- Methyl Ricinoleate - Supplier
- Pentaerythritol Mono Ricinoleate- Manufacturer
- Polyricinoleic Acid
- Ricinoleic Acid
- Butyl Ricinoleate - Supplier
- Calcium Ricinoleate - Manufacturer
- Methyl Acetyl Ricinoleate - Exporter
- Cosmetic Ingredients
- Undecylenic Acid - Cosmetics
- Zinc Ricinoleate
- Potassium Caprylate - Cosmetics
- Cetyl Ricinoleate
- Glyceryl Monoricinoleate
- Heptyl Undecylenate
- Undecanoic Acid - Cosmetics
- Undecylenic Monoethanolamide - Cosmetics
- Zinc Undecylenate - Cosmetics
- Methyl Acetyl Ricinoleate
- Aldehyde C11 Undecylenic - Cosmetics
- Arachidyl Alcohol - Cosmetics
- Behenic Acid Methyl Ester - Cosmetics
- Caprylic Acid - Cosmetics
- Erucic Acid - Cosmetics
- Lauric Acid - Cosmetics
- Methyl Heptanoate - Cosmetics
- Oleic Acid Ethyl Ester - Cosmetics
- Oleyl Alcohol - Cosmetics
- Palmitic Acid - Cosmetics
- Potassium Oleate - Cosmetics
- Sodium Caprylate - Cosmetics
- Soya Oil Methyl Ester - Cosmetics
- Stearic Acid - Cosmetics
- Butyl Undecylenate - Cosmetics
- Castor Oil - Cosmetics
- Glyceryl Mono Undecenoate - Cosmetics
- Heptanoic Acid - Cosmetics
- Linoleic Acid Methyl Ester - Cosmetics
- Palmitic Acid Methyl Ester - Cosmetics
- Sodium Oleate - Cosmetics
- Sodium Undecylenate - Cosmetics
- Stearic Acid Methyl Ester - Cosmetics
- Linoleic Acid Ethyl Ester - Cosmetics
- Emollient
- Cetyl Ricinoleate - Cosmetics
- Glyceryl Mono Undecylenate-Cosmetics
- Glyceryl Monoricinoleate - Cosmetics
- Heptyl Undecylenate - Cosmetics
- Methyl Acetyl Ricinoleate - Cosmetics
- Erucic Acid - Emollient
- Oleic Acid Ethyl Ester - Manufacturer
- Palmitic Acid - Exporter
- Ethyl Undecylenate - Manufacturer
- Glyceryl Mono Undecenoate - Manufacturer
- Palmitic Acid Methyl Ester - Supplier
- Stearic Acid Methyl Ester - Exporter
- Personal Care Ingredients
- Cetyl Ricinoleate - Personal Care Product
- Glyceryl Monoricinoleate- Personal Care Product
- Heptyl Undecylenate - Personal Care Product
- Methyl Acetyl Ricinoleate - Personal Care Product
- Aldehyde C11 Undecylenic - Exporter
- Arachidyl Alcohol - Manufacturer
- Behenic Acid Methyl Ester - Manufacture
- Caprylic Acid - Manufacturer
- Lauric Acid - Personal Care Product
- Oleic Acid Ethyl Ester - Personal Care Product
- Oleyl Alcohol - Manufacturer
- Palmitic Acid - Manufacturer
- Potassium Oleate - Manufacturer
- Soya Oil Methyl Ester - Manufacturer
- Stearic Acid - Manufacturer
- Palmitic Acid Methyl Ester - Manufacturer
- Sodium Oleate - Supplier
- Sodium Undecylenate - Supplier
- Natural Preservatives
- Undecylenates
- Glyceryl Mono Undecylenate-Supplier
- Heptyl Undecylenate Exporter
- Undecanoic Acid 98% Min. By GC - Manufacturer
- Sodium Undecylenate - Manufacturer
- Methyl Undecylenate 98% Min. By GC - Manufacturer
- Undecylenic Acid - Manufacturer
- Zinc Undecylenate - Manufacturer
- Calcium Undecylenate - Manufacturer
- Butyl Undecylenate - Supplier
- Castor Oil - Manufacturer
- Ethyl Undecylenate - Supplier
- Glyceryl Mono Undecenoate - Exporter
- Skin Care Products
- Glyceryl Mono Undecylenate- Skin Care Product
- Zinc Ricinoleate- Skin Care Product
- Glyceryl Monoricinoleate- Skin Care Product
- Heptyl Undecylenate - Skin Care Product
- Calcium Ricinoleate - Supplier
- Methyl Acetyl Ricinoleate - Skin Care Product
- Cetyl Ricinoleate - Skin Care Product
- Aldehyde C11 Undecylenic - Supplier
- Arachidyl Alcohol - Supplier
- Behenic Acid Methyl Ester - Supplier
- Caprylic Acid - Supplier
- Oleic Acid Ethyl Ester - Skin Care Product
- Oleyl Alcohol - Exporter
- Palmitic Acid - Supplier
- Potassium Oleate - Supplier
- Soya Oil Methyl Ester - Supplier
- Stearic Acid - Supplier
- Castor Oil - Supplier
- Glyceryl Mono Undecenoate - Skin Care Product
- Palmitic Acid Methyl Ester - Exporter
- Stearic Acid Methyl Ester - Manufacturer
- Emulsifier
- Glyceryl Monoricinoleate - Emulsifier
- Glyceryl Mono Undecylenate - Emulsifier
- Zinc Ricinoleate - Emulsifier
- Ricinoleic Acid - Emulsifier
- Undecanoic Acid - Emulsifier
- Linoleic Acid - Emulsifier
- Methyl Oleate - Emulsifier
- Oleic Acid - Emulsifier
- Undecylenic Acid - Emulsifier
- Heptaldehyde - Emulsifier
- Silica Gel for Column Chromatography - Emulsifier
- Arachidic Acid - Emulsifier
- Methyl Oleate Commercial- Emulsifier
- Caprylic Acid - Emulsifier
- Lauric Acid - Emulsifier
- Methyl Heptanoate - Emulsifier
- Oleyl Alcohol - Emulsifier
- Palmitic Acid - Emulsifier
- Potassium Oleate - Emulsifier
- Sodium Caprylate - Emulsifier
- Stearic Acid - Emulsifier
- Castor Oil - Emulsifier
- Glyceryl Mono Undecenoate - Emulsifier
- Linoleic Acid Methyl Ester - Emulsifier
- Palmitic Acid Methyl Ester - Emulsifier
- Sodium Oleate - Emulsifier
- Potassium Caprylate - Emulsifier
- Sodium Undecylenate - Emulsifier
- Stearic Acid Methyl Ester - Emulsifier
- Hair Care Products
- Heptyl Undecylenate- Hair Care Product
- Zinc Ricinoleate- Hair Care Product
- Butyl Oleate - Hair Care Product
- Linoleic Acid - Hair Care Product
- Butyl Oleate Commercial -Supplier
- Oleic Acid - Supplier
- Undecylenic Acid - Supplier
- Arachidic Acid - Hair Care Product
- Lauric Acid - Exporter
- Oleic Acid Ethyl Ester - Supplier
- Oleyl Alcohol - Supplier
- Stearic Acid - Exporter
- Sodium Undecylenate - Exporter
- Stearic Acid Methyl Ester - Supplier
- Lubricants
- Glyceryl Monoricinoleate - Lubricant
- Heptyl Undecylenate Lubricant
- Methyl Ricinoleate - Lubricant
- Polyricinoleic Acid - Lubricant
- Pentaerythritol Mono Ricinoleate - Lubricant
- Ricinoleic Acid - Lubricant
- Butyl Oleate - Lubricant
- Butyl Oleate Commercial- Lubricant
- Methyl Undecylenate - Lubricant
- Butyl Ricinoleate - Lubricant
- Methyl Acetyl Ricinoleate - Lubricant
- Behenic Acid Methyl Ester - Lubricant
- Caprylic Acid - Lubricant
- Erucic Acid - Lubricant
- Lauric Acid - Lubricant
- Oleyl Alcohol - Lubricant
- Potassium Oleate - Lubricant
- Soya Oil Methyl Ester - Lubricant
- Stearic Acid - Lubricant
- Castor Oil - Lubricant
- Heptanoic Acid - Lubricant
- Linoleic Acid Methyl Ester - Lubricant
- Palmitic Acid Methyl Ester - Lubricant
- Sodium Oleate - Lubricant
- Stearic Acid Methyl Ester - Lubricant
- Textile Chemicals
- Leather Chemicals
- Pharma Ingredients
- Zinc Undecylenate - Pharma Ingredient
- Undecylenic Acid - Pharma Ingredient
- Caprylic Acid - Pharma Ingredient
- Undecylenic Monoethanolamide
- Calcium Undecylenate - Pharma Ingredient
- Lauric Acid - Pharma Ingredient
- Sodium Caprylate - Manufacturer
- Castor Oil - Pharma Ingredient
- Heptanoic Acid - Supplier
- Sodium Oleate - Manufacturer
- Anti Fungals
- Undecylenic Acid - Antifungal
- Zinc Undecylenate - Anti Fungal
- Undecanoic Acid - Anti Fungal
- Undecylenic Monoethanolamide - Anti Fungal
- Calcium Ricinoleate - Anti Fungal
- Calcium Undecylenate - Anti Fungal
- Caprylic Acid - Anti Fungal
- Lauric Acid - Anti Fungal
- Sodium Caprylate - Anti Fungal
- Castor Oil - Anti Fungal
- Nail Care Product
- Separation Aids
- Perfumery Chemicals
- Alcohol C-11 Undecylenic - Supplier
- Methyl Undecylenate - Exporter
- Heptaldehyde - Manufacturer
- Heptyl Alcohol - Supplier
- Ethyl Undecylenate - Exporter
- Aldehyde C11 Undecylenic - Manufacturer
- Caprylic Acid - Exporter
- Methyl Heptanoate - Supplier
- Oleic Acid Ethyl Ester - Exporter
- Butyl Undecylenate - Manufacturer
- Castor Oil - Perfumery Chemical
- Ethyl Undecanoate - Manufacturer
- Linoleic Acid Methyl Ester - Exporter
- Heptanoic Acid - Manufacturer
- Methyl Undecanoate - Manufacturer
- Linoleic Acid Ethyl Ester - Exporter
- Fragrance Chemicals
- Alcohol C-11 Undecylenic - Exporter
- Heptyl Alcohol - Exporter
- Butyl Oleate - Fragrance Chemical
- Butyl Oleate Commercial - Manufacturer
- Methyl Undecylenate - Perfume & Fragrance Chemical
- Heptaldehyde - Supplier
- Aldehyde C11 Undecylenic - Perfumes & Fragrances
- Caprylic Acid - Perfumes & Fragrances
- Methyl Heptanoate - Perfumes & Fragrances
- Oleic Acid Ethyl Ester - Perfumes & Fragrances
- Castor Oil - Fragrance Chemical
- Ethyl Undecanoate - Supplier
- Ethyl Undecylenate - Perfumes & Fragrances
- Heptanoic Acid - Exporter
- Linoleic Acid Methyl Ester - Supplier
- Methyl Undecanoate - Supplier
- Linoleic Acid Ethyl Ester - Perfumes & Fragrances
- Nutritional Supplements
- Arachidic Acid - Pharmaceutical Chemical
- Elaidic Acid - Pharmaceutical Chemical
- Linoleic Acid - Pharmaceutical Chemical
- Methyl Oleate - Pharmaceutical Chemical
- Oleic Acid - Pharmaceutical Chemical
- Arachidic Acid Methyl Ester - Supplier
- Elaidyl Alcohol - Supplier
- Lauric Acid - Supplier
- Linoleyl Alcohol - Exporter
- Methyl Heptanoate - Manufacturer
- Oleic Acid Ethyl Ester - Pharmaceutical Chemical
- Oleyl Alcohol - Pharmaceutical Chemical
- Sodium Caprylate
- Linoleic Acid Methyl Ester - Manufacturer
- Specialty Fatty Acid
- Arachidic Acid - Manufacturer
- Elaidic Acid - Manufacturer
- Linoleic Acid - Manufacturer
- Methyl Oleate - Manufacturer
- Oleic Acid - Manufacturer
- Methyl Oleate Commercial - Manufacturer
- Arachidic Acid Methyl Ester - Manufacturer
- Arachidic Acid Ethyl Ester - Manufacturer
- Elaidyl Alcohol - Exporter
- Lauric Acid - Manufacturer
- Linoleyl Alcohol - Supplier
- Linoleic Acid Ethyl Ester - Supplier
- Aldehydes
- Silica Gel for Column Chromatography
- Silica Gel for Thin Layer Chroamtography
- Castor Oil Derivatives
- Contact Us

Zinc Undecylenate - Pharma Ingredient
Product Details:
- EINECS No 209-151-9
- Melting Point >200C (decomposes)
- Structural Formula [CH2(CH2)8COO]2Zn
- Solubility Insoluble in water; soluble in mineral acids and alkalies
- Storage Store in a cool, dry place in tightly closed containers
- Molecular Formula C22H38O4Zn
- Particle Size Passes 200 mesh
- Click to view more
X
Zinc Undecylenate - Pharma Ingredient Product Specifications
- 29163990
- White fine powder
- 431.94 g/mol
- Used as an antifungal agent in topical preparations
- Not applicable (decomposes)
- Pharmaceutical Grade
- 5 years
- White
- 557-08-4
- 6.5 - 8.5 (1% suspension)
- Non-poisonous
- Pharma Ingredient
- < 1%
- < 0.002%
- Odorless
- 99%
- 209-151-9
- >200C (decomposes)
- [CH2(CH2)8COO]2Zn
- Zinc Undecylenate
- Insoluble in water; soluble in mineral acids and alkalies
- Store in a cool, dry place in tightly closed containers
- C22H38O4Zn
- Powder
- Passes 200 mesh
- Tasteless
- Zinc undec-10-enoate
Product Description
| Zinc Undecylenate as per BP/EP/USP | |
| PARAMETERS | SPECIFICATIONS |
| Synonyms | Undecylenic Acid Zinc Salt Mycoseptin Tineafax |
| CAS No. | 557-08-4 |
| Formula | [H2C=CH(CH2)8CO2]2 Zn |
| Mol. Wt. | 431.9 |
| Assay as C22H38O4Zn | 98% w/w to 102% w/w |
| Purity | 99% min by GLC. |
| Appearance & Color | A White fine powder. Melts at 120o C. |
| Loss on Drying at 105C | 1.5%max. |
| Sulphate | 500 ppm max. |
| Alkalies & Alkaline Earths | 2.0% Max. |
| Alkalinity | Passes B.P.Test |
| Identification Tests | Positive. |
| Melting Point | 116-121o C |
| Solubility Test | Practically insoluble in water, ethanol(96%) and in ether. |
| Packaging | The material is packed 60 kg net HMHDPE drums open mouth ring with poly bags on the inside |
Acme Synthetic Chemicals is the Manufacturer, Supplier & also the Exporter of Zinc Undecylenate as per BP/EP/USP.
Zinc Undecylenate - Pharma Ingredient is also known as Undecylenic Acid Zinc Salt Mycoseptin Tineafax which is classified under CAS No.557-08-4.
Acme Synthetic Chemicals is one of the reputed organizations engaged in providing superior quality Zinc Undecylenate - Pharma Ingredient to our esteemed clients.
Zinc Undecylenate - Pharma Ingredient, Undecylenic Acid Zinc Salt Mycoseptin Tineafax (CAS No.557-08-4) is used as an active ingredient in powders and ointments for athlete's foot.
Zinc Undecylenate - Pharma Ingredient, Undecylenic Acid Zinc Salt Mycoseptin Tineafax (CAS No.557-08-4) powder meets all requirements of BP, USP and EP.
Zinc Undecylenate - Pharma Ingredient, Undecylenic Acid Zinc Salt Mycoseptin Tineafax (CAS No.557-08-4) is used for its anti-fungal properties.
Zinc Undecylenate - Pharma Ingredient, Undecylenic Acid Zinc Salt Mycoseptin Tineafax (CAS No.557-08-4) is a very economical anti-fungal agent and is used as an active ingredient in many topical over the counter anti-fungal preparations, both powders and ointments.
The Zinc Undecylenate - Pharma Ingredient, Undecylenic Acid Zinc Salt Mycoseptin Tineafax (CAS No.557-08-4) provides an astringent action, reducing rawness and irritation Fungicide.
Zinc Undecylenate - Pharma Ingredient, Undecylenic Acid Zinc Salt Mycoseptin Tineafax (CAS No.557-08-4) act as co-emulsifier in personal care products & as Fungicide, antibacterial agent in medicine.
Zinc Undecylenate - Pharma Ingredient, Undecylenic Acid Zinc Salt Mycoseptin Tineafax (CAS No.557-08-4) is reported to be specially effective for athletes' foot and similar infections.
Our Zinc Undecylenate - Pharma Ingredient, Undecylenic Acid Zinc Salt Mycoseptin Tineafax (CAS No.557-08-4) is manufactured by a group company which has a FDA License and is locally certified as CGMP.
We can supply Zinc Undecylenate - Pharma Ingredient, Undecylenic Acid Zinc Salt Mycoseptin Tineafax (CAS No.557-08-4) as per USP with relevant COA.
Our current capacity for Zinc Undecylenate - Pharma Ingredient (Undecylenic acid Zinc salt, CAS No.557-08-4) is 350 tons per annum.
Zinc Undecylenate - Pharma Ingredient is formulated using supreme class ingredients and modern techniques by our team of skilled professionals as per the set industry norms at our state-of-the-art processing unit.
Zinc Undecylenate - Pharma Ingredient is widely demanded in the international market due to its high effectiveness, eco-friendliness & purity, and is offered in different packaging options to meet the varied needs of our clients. Moreover, we are offering the entire range at an affordable cost to our clients.
Superior Stability and Shelf Life
Zinc Undecylenate upholds consistent quality with a shelf life of 5 years when stored in cool, dry, tightly closed containers. Its stability further guarantees its efficacy as an ingredient in long-lasting pharmaceutical and dermatological applications, minimizing the risk of degradation even under extended storage conditions.
High Pharmaceutical Standards
Manufactured to comply with BP/USP and pharmacopeial microbial limits, this pharmaceutical-grade ingredient meets rigorous industry requirements. Users gain assurance of its safety, efficacy, and purity, essential for dermatological preparations and topical antifungal formulations.
Versatile Application in Topical Formulations
Zinc Undecylenate is selected for its excellent antifungal properties, making it ideal for creams, ointments, and powders designed to treat various skin conditions. Its insolubility in water and compatibility with mineral acids and alkalies allow flexible integration into multiple pharmaceutical formats.
FAQs of Zinc Undecylenate - Pharma Ingredient:
Q: How should Zinc Undecylenate be stored to maintain its stability?
A: Zinc Undecylenate should be stored in a cool, dry place within tightly closed containers to preserve its quality and ensure stability for up to 5 years.Q: What is the primary application of Zinc Undecylenate in pharmaceuticals?
A: It is predominantly used as an antifungal agent in topical preparations, including creams and ointments for dermatological treatments.Q: When is Zinc Undecylenate recommended for use in pharmaceutical production?
A: Zinc Undecylenate is recommended when a high-purity, stable antifungal ingredient is required for pharmaceutical or dermatological product formulations.Q: Where does Zinc Undecylenate find its major market as a pharma ingredient?
A: Its major market includes pharmaceutical manufacturers, suppliers, and traders primarily in India, where it is exported for topical and dermatological uses.Q: What benefits does Zinc Undecylenate offer in topical preparations?
A: The ingredient delivers effective antifungal action, is non-poisonous, odourless, tasteless, and maintains high purity and stability, ensuring safe and consistent results in patient care.Q: How is the quality of Zinc Undecylenate ensured during production?
A: Each batch complies with BP/USP standards and passes pharmacopeial microbial limit tests, guaranteeing pharmaceutical-grade quality and safety for end users.Q: What process should be followed to utilize Zinc Undecylenate in formulations?
A: Manufacturers should measure precise quantities according to pharmaceutical recipes, ensuring the powder passes 200 mesh for uniform distribution in creams or ointments, and account for its insolubility in water when choosing compatible formulation bases.Tell us about your requirement

Price:
Quantity
Select Unit
- 50
- 100
- 200
- 250
- 500
- 1000+
Additional detail
Mobile number
Email




 Call Me Free
Call Me Free
